Abstract
BACKGROUND
Triplet regimens bortezomib + lenalidomide + dexamethasone (VRD) and bortezomib + thalidomide + dexamethasone (VTD) are recommended in both European and US guidelines for the treatment (Tx) of transplant-eligible (TE) patients (pts) with newly diagnosed multiple myeloma (NDMM). To date, there are no randomized controlled trials (RCTs) directly comparing them. Therefore, this integrated analysis evaluated VRD vs VTD in TE pts with NDMM.
METHODS
Published literature was searched for prospective, phase 3 RCTs evaluating VRD or VTD induction (every 3 or 4 weeks) in TE NDMM before autologous stem cell transplant (ASCT). Studies were included if they met pre-defined eligibility criteria, which included having access to pt-level data. The primary objective was noninferiority of the primary endpoint (≥ very good partial response [VGPR] rate post induction. Secondary endpoints included ≥ VGPR rate during induction and post ASCT, and safety. Progression-free survival (PFS) and minimal residual disease (MRD) negativity were exploratory endpoints. Statistical methods for efficacy analyses were based on propensity score (PS), and pts with missing baseline values for the variables used for the PS analyses were excluded. The Cochran-Mantel-Haenszel test, stratified on the stratum based on the quintiles of the PS, was used to estimate the difference of ≥ VGPR rate and 95% CI.
RESULTS
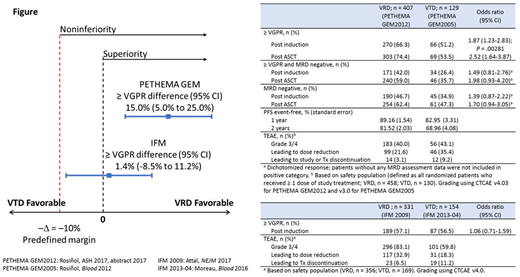
Four studies met the eligibility criteria: VRD, PETHEMA GEM (GEM)2012 and IFM 2009; VTD, GEM2005 and IFM 2013-04. GEM2005 and GEM2012 were the main studies, as they had a symmetrical design of the induction regimens (six 4-week cycles followed by ASCT). IFM studies were considered supportive since they had a variable number of cycles (3 VRD cycles before ASCT vs 8 VRD cycles in IFM 2009 and 4 VTD cycles before ASCT in IFM 2013-04). Thus, IFM analyses used the subgroup of pts from the VRD induction and consolidation arm of IFM 2009 to compare with the IFM 2013-04 VTD arm. No clinically meaningful differences in baseline characteristics were observed between the VRD and VTD PS-stratified cohorts of the GEM and IFM studies.
The integrated analysis met its primary endpoint (noninferiority) and demonstrated a statistically significant and clinically relevant improvement of the ≥ VGPR rate after induction with VRD vs VTD (66.3% vs 51.2%; P = .00281; Figure) in the GEM studies. Non-inferiority results from the IFM studies supported those from GEM, with ≥ VGPR rate by 4 cycles (12 weeks) similar with VRD vs VTD (57.1% vs 56.5%).
In the GEM studies, responses deepened during induction. Among the 378 VRD pts who started cycle 6, ≥ VGPR rate increased from 54.5% by 3 cycles of induction to 62.7% by 4 cycles, and to 70.1% by 6 cycles and post induction. Of the 111 VTD pts, these rates were 35.1%, 40.5%, and 55.9%, respectively. Analyses of ≥ VGPR rate post ASCT (74.4% vs 53.5%) and MRD negativity (10-4) rates post induction (46.7% vs 34.9%) and post ASCT (62.4% vs 47.3%) supported the benefit of VRD vs VTD.
Safety was as previously reported for these studies. Peripheral neuropathy (PN) is a recognized event that can limit VRD and VTD Tx duration. In the GEM studies, subcutaneous (SC) vs intravenous (IV) administration of bortezomib (BORT) may have contributed to lower rates of PN (grouped term) with VRD vs VTD (grade 3/4, 5.5% vs 15.4%; grade ≥ 2, 20.7% vs 44.6%). Treatment-emergent adverse events (TEAEs) led to dose reduction and study or Tx discontinuation less frequently in the VRD vs VTD cohorts, respectively.
In the IFM studies, TEAEs led to dose reduction more frequently and Tx discontinuation less frequently with VRD than with VTD. Grade 3/4 PN (grouped term) was 5.9% vs 15.4%, whereas grade ≥ 2 events were similar (30.3% vs 27.2%), which may reflect BORT administration in these IFM studies (IV for VRD vs SC for VTD, respectively).
CONCLUSIONS
Induction Tx with VRD had a significantly higher ≥ VGPR rate than VTD when 6 cycles of each Tx were compared in TE NDMM. Deepening responses and MRD negativity further support the benefit of VRD over VTD. TEAEs in the GEM and IFM studies were generally consistent with the known safety profiles of lenalidomide, BORT, thalidomide, and dexamethasone. The TEAEs with the VRD regimen were manageable, and the overall tolerability profile compared well with VTD, with lower rates of PN and TEAEs leading to discontinuation. The integrated analysis supports the favorable benefit-risk profile with VRD over VTD as induction Tx in TE pts with NDMM.
Rosinol Dachs:Janssen: Honoraria; Celgene: Honoraria; Amgen: Honoraria. Oriol:Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Rios:Amgen, Celgene, Janssen, and Takeda: Consultancy. Hulin:Celgene: Honoraria, Research Funding; Janssen: Honoraria, Research Funding. Sureda:Merck: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Speakers Bureau; Roche: Honoraria; Sanofi: Honoraria. Mateos:Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Macro:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Financial support for congress; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Financial support for congress; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Financial support for congress; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Financial support for congress. San-Miguel:Amgen: Consultancy; Brystol-Myers Squibb: Consultancy; Celgene: Consultancy; Janssen: Consultancy; MSD: Consultancy; Novartis: Consultancy; Takeda: Consultancy; Sanofi: Consultancy; Roche: Membership on an entity's Board of Directors or advisory committees. Belhadj:Janssen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria. Lahuerta:Amgen: Honoraria; Janssen: Honoraria; Celgene: Honoraria. Chen:Celgene Corporation: Employment. Garelik:Celgene Corporation: Employment. Bladé:Celgene: Honoraria; Amgen: Honoraria; Janssen: Honoraria. Moreau:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal